Total Synthesis of (-)-Alstofolinine A through a Furan Oxidation/ Rearrangement and Indole Nucleophilic Cyclization Cascade
Lei Zhang, Ye Zhang, Wenting Li, and Xiangbing Qi*
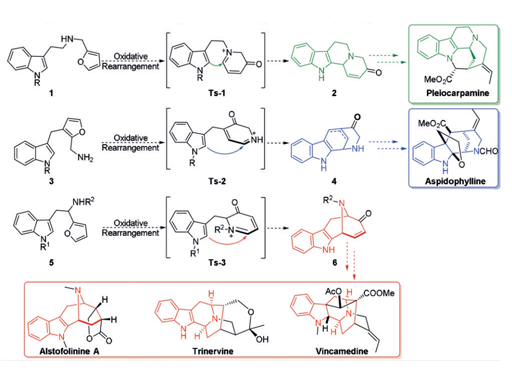
A reaction cascade of aza-Achmatowicz rearrangement followed by indole nucleophilic cyclization was developed to generate the common indole-fused azabicyclo- [3.3.1]nonane core of the macroline family alkaloids. The key to the success of the strategy relies on the careful manipulation of protecting groups and judicious selection of chemoselective furan oxidation conditions. The synthetic utility was further demonstrated on the asymmetric total synthesis of (-)-alstofolinine A.