Development of the Vinylogous Pictet–Spengler Cyclization and Total Synthesis of (±)-Lundurine A
Aaron Nash, Xiangbing Qi, Pradip Maity, Kyle Owens, and Uttam K. Tambar*
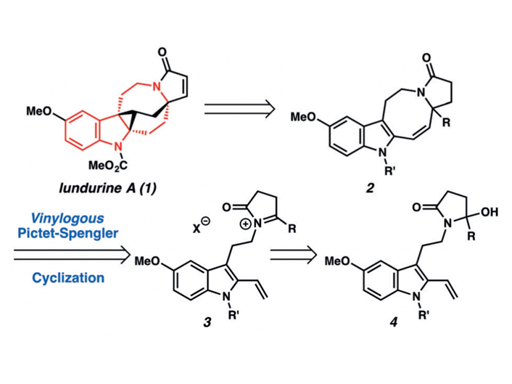
A novel vinylogous Pictet–Spengler cyclization has been developed for the generation of indole-annulated medium-sized rings. The method enables the synthesis of tetrahydroazocinoindoles with a fully substituted carbon center, a prevalent structural motif in many biologically active alkaloids. The strategy has been applied to the total synthesis of (±)-lundurine A.