Estrogen-Related Hormones Induce Apoptosis by Stabilizing Schlafen-12 Protein Turnover
Dianrong Li, Jie Chen, Youwei Ai, Xiaoqiong Gu, Li Li, Di Che, Zhaodi Jiang, Lin Li, She Chen, Huangwei Huang, Jiawen Wang, Tao Cai, Yang Cao, Xiangbing Qi, Xiaodong Wang*
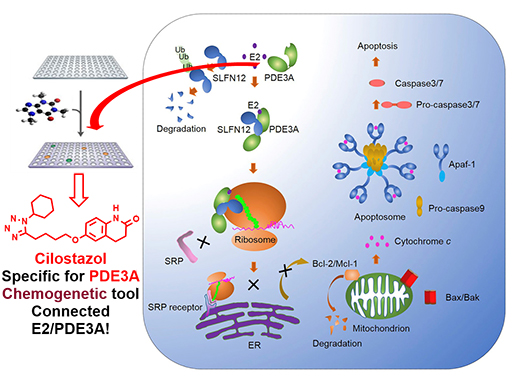
The mitochondrial pathway of apoptosis is controlled by the ratio of anti- and pro-apoptotic members of the Bcl-2 family of proteins. The molecular events underlying how a given physiological stimulus changes this ratio to trigger apoptosis remains unclear. We report here that human 17-β-estradiol (E2) and its related steroid hormones induce apoptosis by binding directly to phosphodiesterase 3A, which in turn recruits and stabilizes an otherwise fast-turnover protein Schlafen 12 (SLFN12). The elevated SLFN12 binds to ribosomes to exclude the recruitment of signal recognition particles (SRPs), thereby blocking the continuous protein translation occurring on the endoplasmic reticulum of E2-treated cells. These proteins include Bcl-2 and Mcl-1, whose ensuing decrease triggers apoptosis. The SLFN12 protein and an apoptosis activation marker were co-localized in syncytiotrophoblast of human placentas, where levels of estrogen-related hormones are high, and dynamic cell turnover by apoptosis is critical for successful implantation and placenta development.
DOI: 10.1016/j.molcel.2019.06.040