A thiazole-derived oridonin analog exhibits antitumor activity by directly and allosterically inhibiting STAT3
Xiaofei Shen 1, Lin Zhao 1, Peihao Chen, Yanqiu Gong, Dingdong Liu, Xia Zhang, Lunzhi Dai, Qingxiang Sun, Jizhong Lou, Zhong Jin, Baohua Zhang, Dawen Niu, Ceshi Chen, Xiangbing Qi, Da Jia
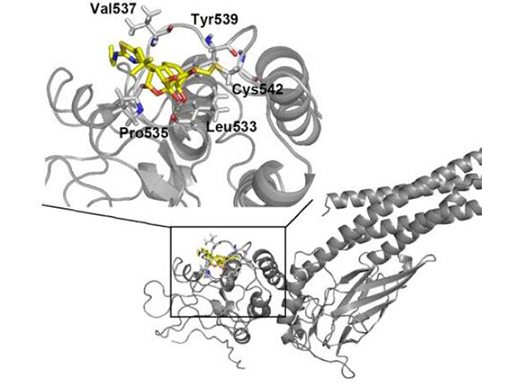
Constitutive activation of signal transducer and activator of transcription 3 (STAT3) occurs in approximately 70% of human cancers, and STAT3 is regarded as one of the most promising targets for cancer therapy. However, specific direct STAT3 inhibitors remain to be developed. Oridonin is an ent-kaurane plant-derived diterpenoid with anti-cancer and anti-inflammatory activities. Here, using an array of cell-based and biochemical approaches, including cell proliferation and apoptosis assays, pull-down and reporter gene assays, site-directed mutagenesis, and molecular dynamics analyses, we report that a thiazole-derived oridonin analog, CYD0618, potently and directly inhibits STAT3. We found that CYD0618 covalently binds to Cys-542 in STAT3 and suppresses its activity through an allosteric effect, effectively reducing STAT3 dimerization and nuclear translocation, as well as decreasing expression of STAT3-targeted oncogenes. Remarkably, CYD0618 not only strongly inhibited growth of multiple cancer cell lines that harbor constitutive STAT3activation, but it also suppressed in vivo tumor growth, via STAT3 inhibition. Taken together, our findings suggest Cys-542 as a druggable site for selectively inhibiting STAT3 and indicate that CYD0618 represents a promising lead compound for developing therapeutic agents against STAT3-driven diseases.