5-IP7: a GPCR messenger mediating muscarinic control of synaptotagmin-dependent insulin exocytosis and glucose homeostasis
Xiaozhe Zhang, Na Li, Jun Zhang, Yanshen Zhang, Xiaoli Yang, Yifan Luo, Bobo Zhang, Zhixue Xu, Zhenhua Zhu, Xiuyan Yang, Yuan Yan, Biao Lin, Shen Wang, Da Chen, Caichao Ye, Yan Ding, Mingliang Lou, Qingcui Wu, Zhanfeng Hou, Keren Zhang, Ziming Liang, Anqi Wei, Bianbian Wang, Changhe Wang, Nan Jiang, Wenqing Zhang, Guozhi Xiao, Cong Ma, Yan Ren, Xiangbing Qi, Weiping Han, Chao Wang,*, and Feng Rao,*
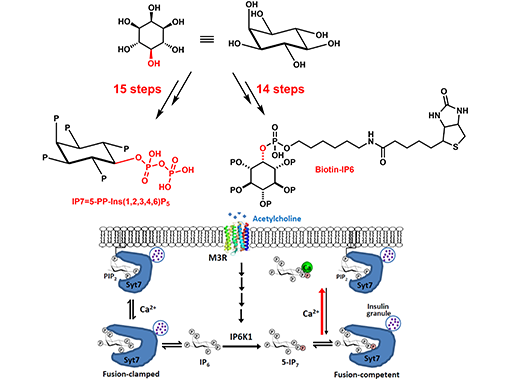
5-diphosphoinositol pentakisphosphate (5-IP7) is a ubiquitous and enigmatic signaling metabolite synthesized by the inositol hexakisphosphate (IP6) kinases (IP6Ks). Here, we show that 5-IP7 participates in neural control of glucose homeostasis by mediating parasympathetic stimulation of synaptotagmin-7 (Syt7)-dependent insulin release. Mechanistically, a muscarinic acetylcholine receptor 3 (M3R)-Gαq-PLC-PKC/PKD axis phosphorylates and activates pancreatic IP6K1 within minutes upon vagal nerve stimulation or upon treatment with the muscarinic agonist carbachol. Both 5-IP7 and its precursor IP6 bind Syt7 in competition with the membrane lipid PIP2. However, Ca2+ selectively binds 5-IP7 with high affinity
thereby freeing Syt7 to promote vesicle membrane interaction. β-cell specific IP6K1 deletion diminishes insulin secretion elicited by vagal nerve stimulation and attenuates glucose clearance induced by carbachol; whereas knockin mice carrying a phosphorylation-mimic, hyperactive IP6K1 mutant display constitutively augmented insulin release, congenital hyperinsulinism and obesity. All of these phenotypes are absent in a Syt7 null background. In light of the evolutionary co-occurrence between IP6Ks and GPCRs, our study suggests a new conceptual framework to understand inositol pyrophosphate physiology: 5-IP7 is a metabolic messenger for GPCR signaling at the interface between peripheral nervous system and metabolic organs, where it transmits G q-coupled GPCR stimulation to unclamp Syt7-dependent and perhaps other exocytotic events.
DOI:10.1038/s42255-021-00468-7