Unlocking the secrets to human NTCP structure
Xiangbing Qi* and Wenhui Li*
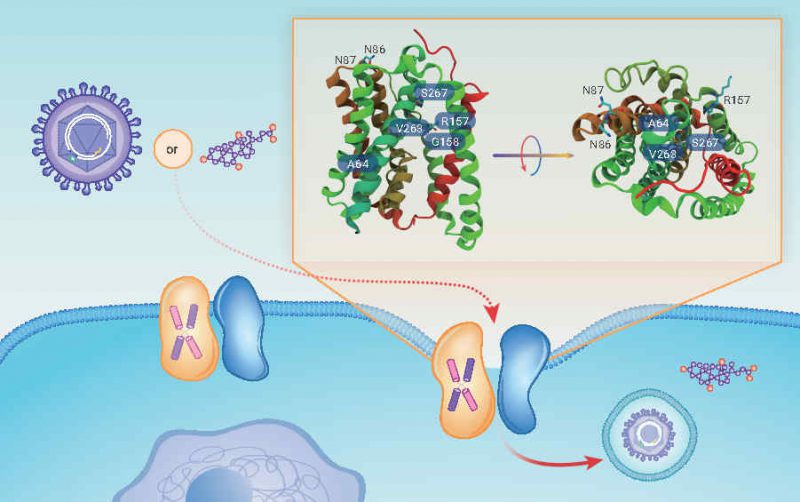
Sodium taurocholate co-transporting polypeptide (NTCP) is encoded by the solute carrier family 10 member 1 (SLC10A1) gene and is predominantly expressed on the sinusoidal membrane of hepatocytes with the function of hepatic uptake of bile salt, steroid hormones, thyroid hormones, and various bile-acid-conjugated drugs. Almost 10 years ago, NTCP was identified as a functional cellular entry receptor of hepatitis B and hepatitis D viruses (HBV/HDV, respectively), which are among the major etiological factors leading to cirrhosis, liver failure, and hepatocellular carcinoma and affect around 250 million people worldwide. Previous studies have demonstrated that the binding site of HBV with NTCP is within the first 48 amino acid residues of the N-myristoylated preS1 (myrpreS1) domain of the large envelope glycoprotein. Genetic variations, such as amino acids 84–87 and 157–165 in the NTCP sequences, are responsible for susceptibility to HBV in different species. Individuals who carry the NTCP p.S267F polymorphism on both alleles are associated with increased resistance to chronic HBV infection. Biochemical and virology studies have shown common molecular determinants as well as function separation on the transporting of bile acids and mediating HBV/HDV infection. However, important questions remain unanswered: for example, the binding mode of bile acid or the preS1 lipopeptide to NTCP on the hepatocyte surface, how to differentiate the pathway of substrate uptake and virus infection, and the dynamics of substrate uptake and the molecular mechanisms of virus infection are all unknown.