Visible-light-induced Enantioselective Radical Cross-Coupling of C(sp3)–Borazirconocene
Chao Yang, Songlin Bai, Yadong Gao, Qingcui Wu, Xiangbing Qi*
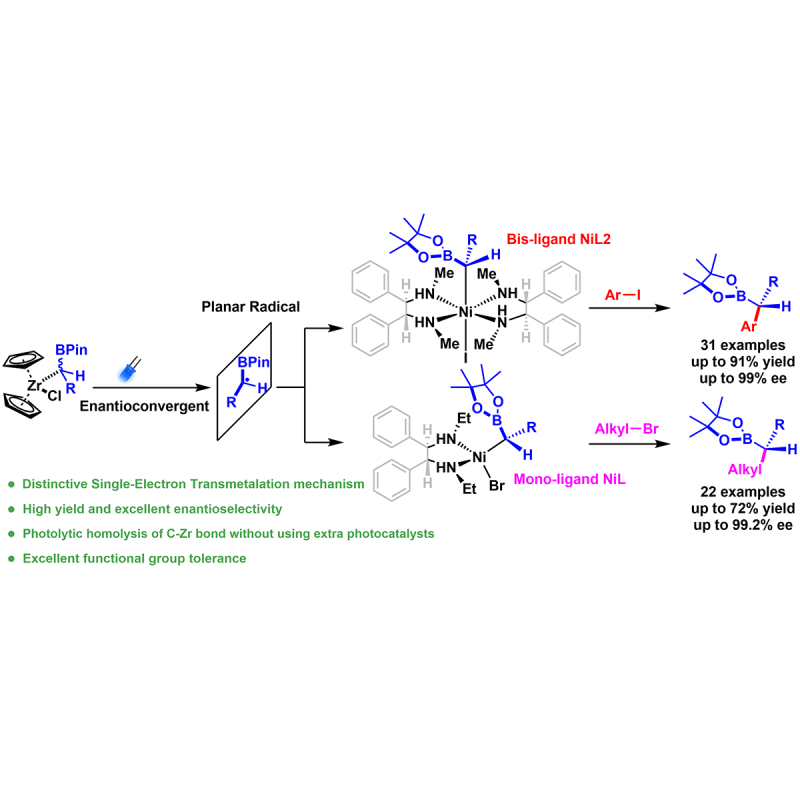
Asymmetric cross-coupling involving sp3-hybridized carbon nucleophiles has become one of the most powerful methods for the synthesis of chiral C(sp3)-enriched three-dimensional (3D) structures, which are essential scaffolds for pharmaceuticals, agrochemicals, and functional materials. To date, in contrast to the application of several alkyl organometallic nucleophiles (e.g., alkyl-zinc, boron, and magnesium) in asymmetric cross-couplings, more easily accessible alkylzirconocene reagents have received much less attention. Herein, we report a visible-light-induced, Ni-catalyzed, asymmetric cross-coupling of gem-borazirconocene alkenes with aryl and unactivated alkyl halides, furnishing a diverse series of valuable chiral alkylboron compounds in good yield and with excellent enantioselectivity. Additionally, mechanistic investigations, including DFT calculations, radical trapping and kinetic experiments, show how the inherent photoreactivity of alkyl-Zr controls the radical generation step that is essential for cross-coupling, unveiling an efficient means for highly stereoconvergent, gem-borazirconocene alkene-based, single-electron asymmetric transformations.