A brief overview of classical natural product drug synthesis and bioactivity
Gen Li, Mingliang Lou and Xiangbing Qi
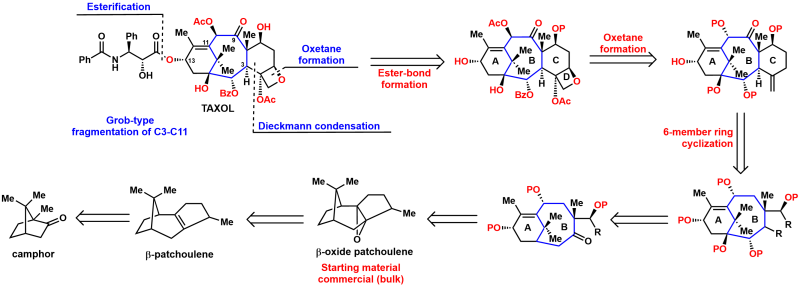
Traditional medicines consisting of compounds derived from natural organisms have been used for human health care worldwide since ancient times. Since the last century, huge numbers of bioactive natural entities with diverse chemical scaffolds have been discovered, and some have been explored as clinical medications to treat various diseases. The advent of modern technologies has promoted the discovery of natural product-based pharmaceutical agents. The synthesis of natural products not only paves the way to confirm their molecular structures but also offers the structural modification opportunity to rationally optimize the drug-likeness parameters and evaluate the bioactivity of analogs. By providing a brief overview of a miscellaneous collection of complex natural products synthesis and the efforts of the structure–activity relationship, the present report aims to highlight the impact of chemical synthesis in natural product generation, diversification, bioactivity evaluation, and natural product-based drug development.
DOI: 10.1039/D1QO01341F