Radical Ring Expansion Catalyzed by an α-Ketoglutarate-Dependent Dioxygenase in the Biosynthesis of Tropolones
Mingjie Liu, Jiawang Liu, Songlin Bai, Ziang Chen, Yaling Wu, Dan Du, Xiangbing Qi, Xiaojing He and Youcai Hu
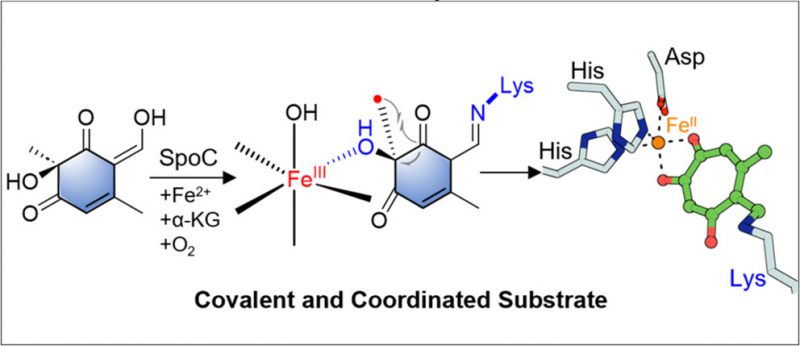
Tropolones are the core of numerous remarkable bioactive natural products, such as stipitatic acid and eupenifeldin. The key step of the biosynthetic pathways of tropolones is the ring expansion of a six-membered ring. TropC is the first identified enzyme capable of catalyzing the tropolone ring expansion process, which belongs to the α-ketoglutarate-dependent dioxygenase (α-KGD) family. However, the molecular mechanism underlying this enzymatic process remains elusive. Here, we present biochemical evidence indicating that SpoC, an enzyme closely related to TropC, also catalyzes ring expansion to construct the tropolone core with stringent catalytic specificity. Molecular insight into the oxidative rearrangement mechanism of SpoC has been demonstrated through stable isotope labeling, protein mass spectrometry, crystallographic analyses, mutagenesis studies, and quantum mechanical calculations. These findings support an intriguing radical ring expansion mechanism facilitated by imine-assisted monodentate coordination of the substrate to an oxo-iron center, enabling an efficient radical ring expansion reaction. This understanding is essential for the synthesis of various tropolone-containing natural products, pharmaceuticals, and functional materials with unique properties.
DOI: 10.1021/jacs.5c11241