Total Synthesis of Tetracyclic Spirooxindole Alkaloids via a Double Oxidative Rearrangement/Cyclization Cascade
Xin Wang, Mengjiao Zhang, Xiaolei Liu, Mingliang Lou, Gen Li and Xiangbing Qi
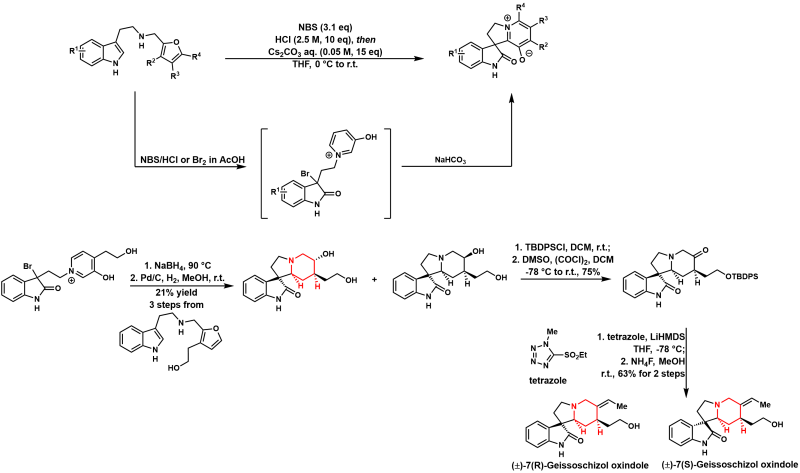
Skeleton rearrangement could rapidly transfer simple molecules to complex structures and has significant potential in the total synthesis of natural products. We developed a one-pot reaction cascade of double oxidative rearrangement of furan and indole followed by a nucleophilic cyclization that was successfully applied for the formal synthesis of rhynchophylline/isorhynchophylline and the first total synthesis of (±)-7(R)-geissoschizol oxindole/(±)-7(S)-geissoschizol oxindole. In addition, the geissoschizol oxindoles were revised to their C3 epimers, and the mechanism for the reversed stereochemistry through the retro-Mannich/Mannich cascade was proposed and supported by density functional theory calculations.