Triflic Anhydride Promoted Decarboxylative Functionalization of α-Amino Acids
Mingyue Yuan, Mingliang Lou, Gen Li and Xiangbing Qi
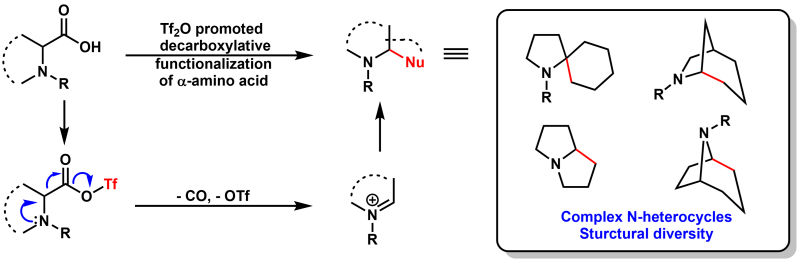
The decarboxylation of naturally abundant amino acids, followed by subsequent inter- or intramolecular reaction cascades, enables the rapid synthesis of a variety of diverse and high-value amine derivatives. Previous methods have relied heavily on transition metals, involved tedious procedures, or required harsh conditions. Herein, we present a novel reaction cascade for the decarboxylation and nucleophilic functionalization of α-amino acids. This method is characterized by being transition-metal-free, convenient to operate, environmentally friendly and having mild conditions.