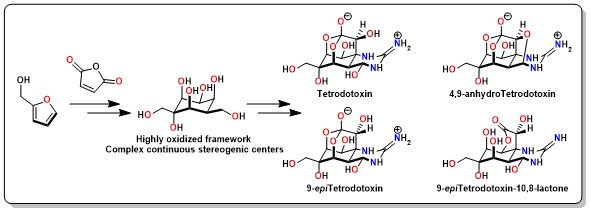
Total Synthesis of Tetrodotoxin and 9-epiTetrodotoxin
Peihao chen ,Jing Wang ,Yan Wang ,Yuze Sun ,Songlin Bai ,Qingcui Wu ,Shuangfeng Zhang ,Xinyu Cheng ,Peng Cao ,Xiangbin Qi
Tetrodotoxin and congeners are specific voltage-gated sodium channel blockers that exhibit remarkable anesthetic and an-algesic effects. Here, we present a scalable asymmetric synthesis of TTX and 9-epiTTX from the abundant chemical feed-stock furfuryl alcohol. The optically pure cyclohexane skeleton was assembled via a stereoselective Diels-Alder reaction. The dense heteroatom substituents were established sequentially by a series of functional group interconversions on highly oxygenated cyclohexane frameworks, including a chemoselective cyclic anhydride opening, and a decarboxylative hydrox-ylation. An innovative SmI2-mediated concurrent fragmentation, an oxo-bridge ring opening and ester reduction followed by an Upjohn dihydroxylation delivered the highly oxidized skeleton. Ruthenium-catalyzed oxidative alkyne cleavage and formation of the hemiaminal and orthoester under acidic conditions enabled the rapid assembly of TTX, anhydro-TTX, 9-epiTTX, and 9-epi lactone-TTX.