Cp*Ir(III)-Catalyzed Asymmetric Tandem Intramolecular Aziridination/Aza-Semipinacol Rearrangement of Alkenyl 1,4,2-Dioxazol-5-one
Mengbo Wu, Yuanzhen Xu, Songlin Bai, Qiqi An, Jiacheng Song, Pengpeng Gao, Jian Li, Jiyou Huo, Xiangbing Qi, Jiahang Yan and Weiqing Xie
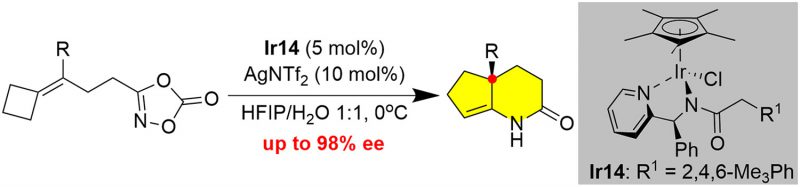
Aza-semipinacol rearrangement provides facile access to the quaternary carbon center adjacent to imine or enamine. Despite the enormous advances that have been witnessed during the last two decades, the catalytic asymmetric aza-semipinacol has been far less explored. In this study, we disclose an innovative [Cp*IrCl2]2-catalyzed tandem aziridination/aza-semipinacol rearrangement of alkenyl 1,4,2-dioxazol-5-one, facilitating the synthesis of cyclopenta[b]pyridin-2-ones with a quaternary carbon center. Furthermore, we successfully achieved Cp*Ir(III)-catalyzed asymmetric tandem aziridination/aza-semipinacol rearrangement of alkenyl 1,4,2-dioxazol-5-one, using 2,4,6-trimethylphenylacyl tailored chiral 2-(aminomethyl)pyridine as the ligand. By taking advantage of this novel chiral catalyst, bicyclic lactams were obtained with good to excellent enantioselectivities via a catalytic asymmetric cascade. Density functional theory (DFT) calculations suggest that the reaction is initiated with the generation of an Ir-acyl nitrenoid, which undergoes enantioselective intramolecular aziridination under the influence of the chiral ligand. The ensuing aza-semipinacol rearrangement driven by the release of ring strain leads to enantioenriched bicyclic lactams via a stereospecific rearrangement.