Visible-Light-Induced Nickel-Catalyzed Cross-Coupling with Alkylzirconocenes from Unactivated Alkenes
Yadong Gao, Chao Yang, Songlin Bai, Xiaolei Liu, Qingcui Wu, Jing Wang, Chao Jiang* and Xiangbing Qi*
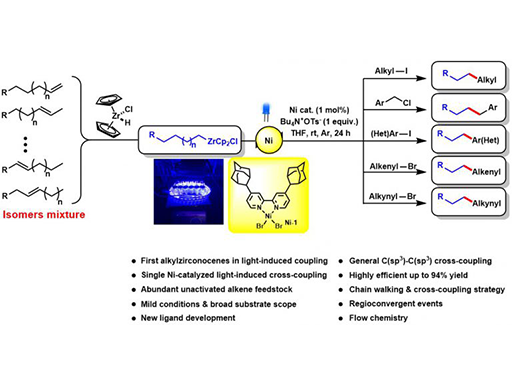
Transition-metal-catalyzed cross-coupling reactions between naturally abundant sp3-hybridized carbon centers facilitate access to diverse molecules with complex three-dimensional structures. Organometallic compounds are among one of the most powerful reagents that are broadly used in carbon–carbon bond formations. Although sp2-hybridized organometallic compounds are widely employed in cross-couplings, sp3-hybridized organometallic coupling partners are less developed. Herein, we report visible-light-induced single nickel-catalyzed C(sp3)–C(sp3), C(sp3)–C(sp2), and C(sp3)–C(sp) cross-coupling reactions using alkylzirconocenes, which are easily generated in situ from terminal or internal unactivated alkenes through hydrozirconation and chain walking. This method is mild and applicable for a large range of substrates including primary, secondary, tertiary alkyl, aryl, alkenyl, alkynyl halides, and a variety of alkenes. Mechanistic studies suggest a novel nickel-catalyzed radical crosscoupling pathway, which represents the first visible-light-induced transformation of alkylzirconocenes.