A general strategy for synthesis of cyclophanebraced peptide macrocycles via palladiumcatalysed intramolecular sp3 C−H arylation
Xuekai Zhang, Gang Lu, Meng Sun, Madhu Mahankali, Yanfei Ma, Mingming Zhang, Wangde Hua, Yuting Hu, Qingbing Wang, Jinghuo Chen, Gang He*, Xiangbing Qi *, Weijun Shen*, Peng Liu * and Gong Chen *
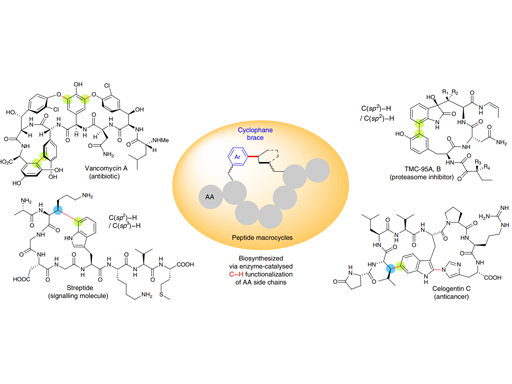
New methods capable of effecting cyclization, and forming novel three-dimensional structures while maintaining favourable physicochemical properties are needed to facilitate the development of cyclic peptide-based drugs that can engage challenging biological targets, such as protein–protein interactions. Here, we report a highly efficient and generally applicable strategy for constructing new types of peptide macrocycles using palladium-catalysed intramolecular C(sp3)–H arylation reactions. Easily accessible linear peptide precursors of simple and versatile design can be selectively cyclized at the side chains of either aromatic or modified non-aromatic amino acid units to form various cyclophane-braced peptide cycles. This strategy provides a powerful tool to address the long-standing challenge of size- and composition-dependence in peptide macrocyclization, and generates novel peptide macrocycles with uniquely buttressed backbones and distinct loop-type three-dimensional structures. Preliminary cell proliferation screening of the pilot library revealed a potent lead compound with selective cytotoxicity toward proliferative Myc-dependent cancer cell lines.